Our website is not supported on this browser
The browser you are using (Internet Explorer) cannot display our content.
Please come back on a more recent browser to have the best experience possible
The medical device industry has unique challenges due to its segmentation (product nature and geography), high quality demand, and irregular supply chain variations. Nevertheless, its market value is estimated to grow over 25% in the next 5 years alone (source). To make the most of this ramping market rise, companies need to place themselves in the wining position by applying the best PM techniques and ensuring the best value is delivered at each stage of the journey.
In this article, we’ll explore the unique characteristics of the medical device industry on its several markets and how hybrid (Agile + Waterfall) PM techniques are key to a successful delivery.
Medical devices are used in a multitude of scenarios ranging from patient diagnostic, prevention or recovery. They are often technologically advanced and fit for a specific purpose or situation.
Their widespread use projects a continuous growth, with the global medical devices market being valued at USD 488.98 billion in 2021 and an expected compound annual growth rate of 5.5% until 2029.
These numbers are fuelled by the expected growth of chronic disorders, such as diabetes, cancer and other infectious diseases due to both an increasingly sedentary lifestyle in developed countries as well as the push for diagnosis and treatment rates from healthcare agencies of various emerging economies.
Understanding the market unique traits is key to a successful delivery.
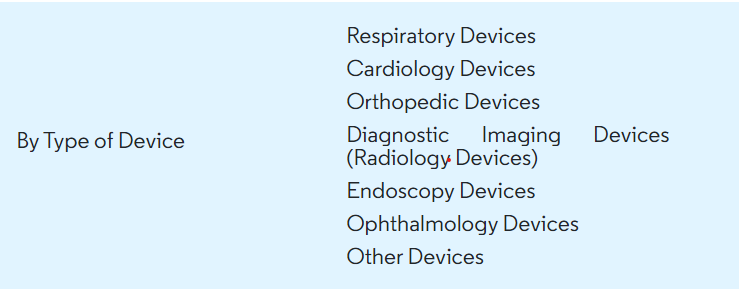
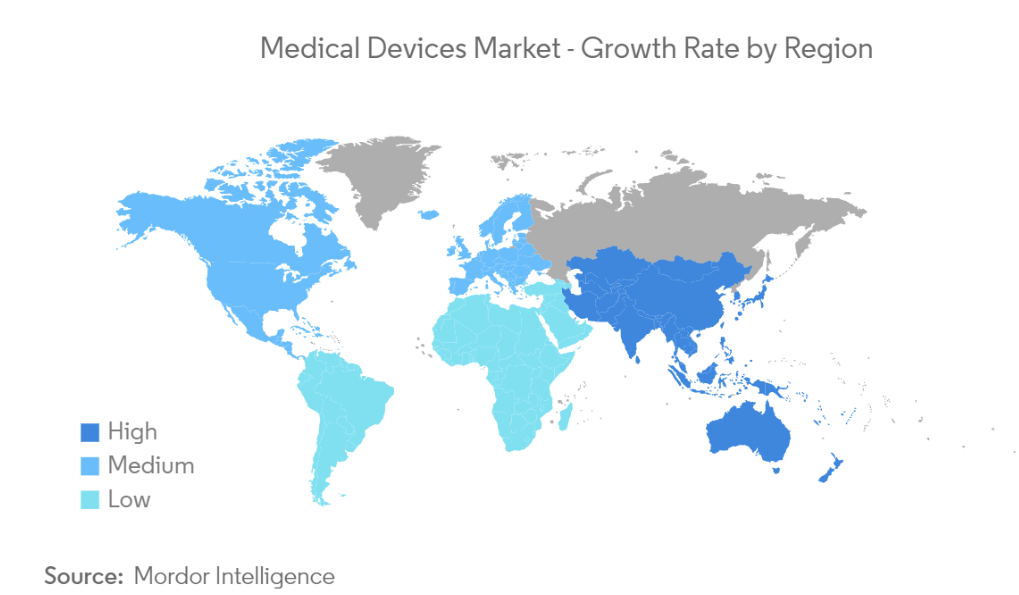
Laying the groundwork, medical devices are mainly divided by type and geography (fig. 1 and fig. 2) Knowing where you stand within this map will help companies navigate the best outcome as different regulatory compliances, distribution and supply chain awareness and market analysis, all must be considered (source).
In addition to type and geography, there medical devices are also defined by risk level, typically ranging from minimum to high or maximum based on the level of risk they pose to patients: Class I devices have the lowest level of risk, Class II devices have a moderate level of risk, and Class III devices have the highest level of risk. The level of risk associated with a device also plays a significant role in determining the regulatory pathway that a device must follow to gain approval for commercial use.
There is no easy process in the medical device industry. Given the multiple classifications, complexity of legal steps and implementation (i.e., putting a medical device into practical use or making it available for its intended purpose) in different areas, it when considering what methodology an organisation needs to implement in order to adhere to regulations.
In the past decade there have been regarding new technologies and design modifications, these devices are expensive, with subsequent high acquisition and maintenance costs. Some devices are associated with various other components such as chips, batteries, sensors and other accessories, which require periodic replacement. As the demand for medical device continues so does the need to manage the process effectively.
There is no easy process in the medical device industry. Given the multiple classifications, complexity of legal steps and implementation (i.e., putting a medical device into practical use or making it available for its intended purpose) in different areas, it when considering what methodology an organisation needs to implement in order to adhere to regulations.
In the past decade there have been regarding new technologies and design modifications, these devices are expensive, with subsequent high acquisition and maintenance costs. Some devices are associated with various other components such as chips, batteries, sensors and other accessories, which require periodic replacement. As the demand for medical device continues so does the need to manage the process effectively.
When using a hybrid project management methodology in the medical devices industry, it’s important to consider additional factors that can impact project success. These include using risk management as a proactive tool to reduce risk and drive improvements, as well as effective stakeholder management and cross-functional team collaboration. Building a high-performing agile team is also critical, and organisations should prioritise knowledge sharing and coaching to ensure that all team members have the necessary skills and expertise. Finally, project managers should champion early cross-functional planning in project and product life cycles to ensure that all stakeholders are aligned and that project goals are achieved. By taking these considerations into account, medical device organisations can maximise the benefits of a hybrid project management methodology and successfully deliver products that meet both customer needs and regulatory requirements.

So, how can the right project management techniques generate value amidst the settled scenario? On one hand, traditional Waterfall approaches are needed when navigating the legal documentation required by quality regulators at different stages of the delivery process. This, however, comes with the well-known hindrance that products don’t get fully looked at until each step in the chain has been completed. This then requires costly rework and re-design of medical devices.
On the other hand, the typical approach that we usually see in software development companies of agile has expanded broadly more into the pharmaceutical projects. There’s been a high interest in implementing an adaptive agile approach which allows you to catch problems before it becomes too late, encouraging regular user feedback and regulatory feedback along the way. This also means that it is easier and cheaper for a company, particularly in the product development phase.
Due to rapid changing product cycles, it can become extremely difficult to keep up with the necessary documentation. Thus, we see companies adopting the hybrid approach combining elements of both waterfall and agile. Balancing the best of both worlds keeping up with the documentation/structure but gaining the valuable user feedback along the way.
In the past, the life sciences industry predominantly followed traditional waterfall approaches due to the strict regulatory requirements, documentation needs, and quality assurance standards. However, in recent years, there has been an increasing recognition of the benefits of agile methodologies in this industry as well. Currently regulators have adapted to the current trait and allow for companies or sponsors to choose which project management methodology to use as long as it suits the regulatory requirements.
When using a hybrid project management methodology in the medical devices industry, it’s important to consider additional factors that can impact project success. These include using risk management as a proactive tool to reduce risk and drive improvements, as well as effective stakeholder management and cross-functional team collaboration. Building a high-performing agile team is also critical, and organisations should prioritise knowledge sharing and coaching to ensure that all team members have the necessary skills and expertise. Finally, project managers should champion early cross-functional planning in project and product life cycles to ensure that all stakeholders are aligned and that project goals are achieved. By taking these considerations into account, medical device organisations can maximise the benefits of a hybrid project management methodology and successfully deliver products that meet both customer needs and regulatory requirements.
By adopting an agile approach to project management, teams can catch issues quickly and address them in real-time, reducing the need to re-do work and minimising the risk of delays or cost overruns. This approach also enables continuous improvement, allowing teams to refine their processes and deliver higher quality products over time. In addition, quality assurance is built into the process, with rigorous testing and validation at every stage of development. Finally, by sticking to deadlines and delivering on time, organisations can improve customer satisfaction and maintain a competitive edge in the market.
In conclusion, the medical devices industry is going through an exciting time of innovation, with new devices and products emerging that have the potential to benefit patients in numerous ways. However, this innovation is also occurring in the context of increasing regulatory scrutiny worldwide, which places additional demands on the development and delivery of these products.
To successfully navigate these challenges, organisations must adopt the right approach to project management. In the medical devices industry, this often involves a hybrid project management methodology that combines the strengths of agile and waterfall approaches. This allows teams to be flexible and responsive to changing requirements, while also ensuring that projects are delivered on time, within budget, and in compliance with regulatory requirements.
Agile teams are essential to the success of medical devices projects, as they allow for rapid prototyping, iterative testing, and close collaboration between cross-functional teams. By using a hybrid project management methodology that incorporates agile principles, organisations can maximise earned value and achieve their project goals, while also staying competitive in an industry that demands innovation and speed.
This article was written by Gastao Beaumont, Consultant.
Loved what you just read?
Let's stay in touch.
No spam, only great things to read in our newsletter.
We combine our expertise with a fine knowledge of the industry to deliver high-value project management services.
MIGSO-PCUBED is part of the ALTEN group.
Find us around the world
Australia – Canada – France – Germany – Italy – Mexico – Portugal – Romania – South East Asia – Spain – Switzerland – United Kingdom – United States
© 2024 MIGSO-PCUBED. All rights reserved | Legal information | Privacy Policy | Cookie Settings | Intranet
Perfect jobs also result from great environments : the team, its culture and energy.
So tell us more about you : who you are, your project, your ambitions,
and let’s find your next step together.
Dear candidates, please note that you will only be contacted via email from the following domain: migso-pcubed.com. Please remain vigilant and ensure that you interact exclusively with our official websites. The MIGSO-PCUBED Team
Choose your language
Our website is not supported on this browser
The browser you are using (Internet Explorer) cannot display our content.
Please come back on a more recent browser to have the best experience possible
